When I explain my dissertation project, I usually have to explain it in two parts. First I explain the problem, and then I explain the possible solution. In this case, the possible solution is a technology called electron beam (or eBeam) technology.
eBeam is a form of ionizing radiation created from commercial electricity. Now, before I go any further, I know most people hear radiation and think of Chernobyl. But there are a lot of good things that radiation can do! And, not all radiation is the same.
Ionizing Radiation


Radiation can be thought of as the transmission of energy in the form of waves. The number of waves that pass by a point in a given amount of time is referred to as the wave frequency. A high frequency corresponds to a high energy. A good way to think about this is as if you were jumping rope. In order to make the rope turn faster, you have to put more energy in.
I am focused on a subset of radiation known as ionizing radiation. Ionizing radiation refers to energy on the the upper end of the electromagnetic spectrum which has a higher energy and higher frequency than visible light or microwaves. Similar to the graphic on the right, ionizing radiation refers to energy that is high enough to be able to knock electrons out of their orbit around an atom or molecule. When this occurs, the atom is referred to as ionized (clever right?). Normally, atoms like to be ‘balanced’, so, when an atom is ionized it often becomes unbalanced and carries a negative or positive charge. This also makes that atom or molecule more reactive, which can lead to a chain of further reactions with other atoms.
Types of Ionizing Radiation
However, like the electromagnetic spectrum graph suggests, there are multiple types of ionizing radiation. These can be separated based upon the particle that is causing radiation. These are:
- Alpha (α)
- Beta (β)
- X-ray
- Gamma (γ)
- Neutrons
Mirion technologies has a good article explaining the differences between all of these particles, but I am just going to focus on those relevant to what I do: Beta, X-ray, and Gamma.
Gamma irradiation is the type of irradiation most people normally think about. It is caused when a radioactive element decays (like cobalt-60 or cesium-137).
Atoms are made up of protons (+ charged particles), neutrons (neutral charged particles), and electrons (- charged particles). Protons and neutrons are found in the center of the atom and together are called the nucleus. Isotopes are atoms that have the same number of protons and electrons, but a different number of neutrons. Radioactive elements are isotopes that are unstable due to this change in neutrons.
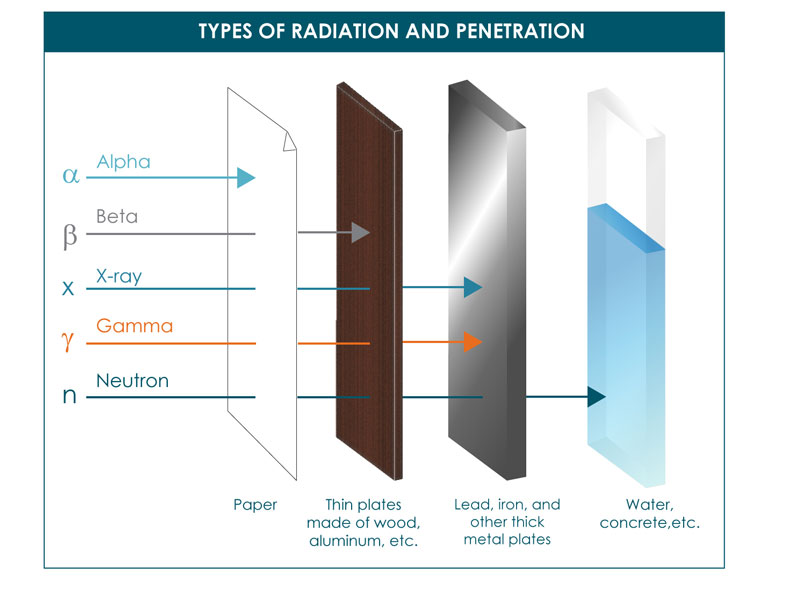
Radioactive elements undergo decay which means they release energy to try to re-stabilize the atom. But this often happens very slowly which is why many radioactive elements are dangerous and hard to get rid of. Gamma irradiation is the main form of energy that is released from these elements and this energy is released in the form of a photon. Photons do not have a charge or mass which makes them highly penetrable.
X-rays are similar to gamma rays and are also made up of photons. The difference is the way that they are generated. Gamma energy originates from the nucleus of the unstable isotope. X-rays however, originate from energy changes of the electrons orbiting the nucleus. Commercially, X-rays can also be created without the use of radioactive elements. Instead, electricity can be used with heavy atomic elements such as tantalum to create X-rays. When you get a medical X-ray, your bones contain calcium, which is much more dense than other tissues. So, when you get an X-ray your bones are able to block some of the irradiation leading to ‘shadows’ that show up on the film.
Finally, what I am interested in are beta particles. Beta particles, in my case, are energy in the form of electrons themselves. Electrons do have a very small mass (unlike photons) and are negatively charged. This means that they penetrate less deeply into materials. However, similarly to X-rays, they can be produced using electricity instead of dangerous radioactive sources. When produced this way, we refer to it as electron beam irradiation.
Electron Beam Technology
Now that you understand what ionizing radiation is, I can explain why it is so cool. eBeam irradiation technology is the form of ionizing radiation technology I’ve been working with during my PhD at Texas A&M with the National Center for Electron Beam Research (NCEBR).
How does it work?

Like I mentioned above, eBeam irradiation can be produced from commercial electricity. The beam of electrons is created when the electrons in electricity are accelerated to 99.99% the speed of light using an accelerator. There are multiple types of accelerators but NCEBR uses a linear accelerator (pictured to the right). Once the electrons are accelerated, they are released through a magnetic “scan horn” which allows us to focus them into a beam to treat products.
We refer to the amount of eBeam irradiation a product receives as the dose and we measure it in a unit called kilograys (kGy). The dose is a result of how quickly the product moves through the beam. At NCEBR, we treat products on a conveyor belt. So, the speed of the conveyor belt dictates the dose that a product receives.
There are two major qualities that set eBeam apart from gamma technologies. The first is that eBeam does not require a radioactive source. Using radioactive materials are dangerous because they are unstable and constantly emitting radiation. Because eBeam is electricity derived, it is able to be turned on and off. The second quality is that eBeam allows for more control over dosing. eBeam has a much higher dose rate, or energy delivered per second, meaning that higher doses can be reached faster than gamma or X-ray technologies. eBeam also allows for more dose flexibility. Gamma irradiation is dependent upon the decay of the radioactive element to determine how much dose is being applied to a product. Because we can control the speed the product passes through the beam, we always control the dose applied to the product.
What is it used for?

All three of the forms of irradiation I mentioned are utilized in many areas of industry. Particularly, gamma irradiation and eBeam irradiation technologies are used to sterilize medical equipment, sanitize packaged foods and fresh produce, cross-link cables and polymers, and even to change the colors of gemstones. Fun fact, the Food and Drug Administration (FDA) requires that irradiated foods bear the radura symbol like that on the right. Check for this next time you’re in the grocery store! More recently, these technologies are being studied for use in breaking down pollutants in the environment (this is where I come in).
But why can eBeam be used in these ways? Well, there’s two major ways that eBeam is effective at breaking down pollutants and inactivating pathogens.
- Direct damage – this refers to damage caused by the irradiation itself. The energetic electrons can interact with chemicals or the DNA in an organism to break them apart.
- Indirect damage – this refers to damage caused by reactive species that were caused by the initial direct damage. Often during eBeam irradiation, the beam causes radiolysis of water. Radiolysis of water is a process where water molecules are broken up into reactive versions of themselves. These reactive molecules can then do further damage to pollutants or microorganisms.
What do I use it for?
In my previous dissertation miniseries post, I explained that harmful cyanobacterial blooms (HCBs) are increasing in occurrence and responsible for producing various toxins. These blooms are occurring in water bodies that we use to supply our drinking water. This means that water treatment plants must remove the cyanobacteria and their toxins to prevent them from getting into your tap.
My project focuses on understanding how eBeam irradiation could be used in the water treatment process to breakdown these cyanotoxins and cyanobacteria. Using eBeam, I hope to understand how a cyanobacteria cell reacts to irradiation as well as if we can degrade the toxin itself to make it non-toxic.
Stay tuned for more of the dissertation miniseries to get a glimpse at what I research in my PhD and why I use electron beam technology!
If you’re interested in learning more, check out these websites and scientific articles linked below!
- National Center for Electron Beam Research
- FDA – Food Irradiation: What you need to know
- Food Irradiation Across the World
- Environmental Applications of Ionizing Radiation – Chmielewski, 2005
- Electron Beam for Potable Water Reuse – Wang et al., 2016




1 COMMENT
Elaine Jagers
3 years agoGreetings I am so happy I found your site, I really found you by accident, while I was searching on Aol for something else, Nonetheless I am here now and would just like to say thanks a lot for a fantastic post and a all round entertaining blog (I also love the theme/design), I don’t have time to read through it all at the moment but I have saved it and also included your RSS feeds, so when I have time I will be back to read a great deal more, Please do keep up the superb work.