When we talk about getting tested for SARS-CoV-2 (aka. the virus that causes COVID-19) what does this mean? How do we test whether or not you have been exposed to the virus? You also may have heard that new rapid tests have been developed. What is the difference between these tests and the ‘slower’ ones?
Like I mentioned in my Bacteria vs. Viruses blog post, the main way to differentiate between a bacterial or a viral infection is through testing. This is because both bacterial and viral infections can have similar symptoms. Also, some people can have different symptoms even with the same infection, like we are seeing with COVID-19 (1). Tests for COVID-19 can be broken into two main categories: genetic based tests and antibody based tests. I’ll break them down in some detail below.
Note: This is a long article! I summarize the takeaways at the end.
Genetic Based Tests
Genetic based tests are currently the most common tests being used to diagnose COVID-19 in the United States. Although whether a virus is alive or not is highly debated, viruses still contain genetic material that allows them to function.
The Technology
These genetic based tests utilize what is called real-time reverse transcriptase polymerase chain reaction (rRT-PCR). Regular PCR is a method that was developed in 1985 in order to make millions of copies of DNA from a sample that only contains a small amount of DNA. Normally, PCR is used to target a specific region of DNA and only this region gets amplified in the process. This region of amplification is chosen with the use of primers. Primers are just short sequences of genetic material that correspond to the region of DNA that you want to copy. PCR is done in three basic steps:
- Denaturation: The DNA is heated to separate the strands of DNA (remember DNA is double stranded).
- Annealing: The now single stranded DNA is cooled so that primers can bind to where they match on the DNA.
- Extension: The temperature of the reaction is raised again so that enzymes can ‘build’ new strands of DNA starting from where the primers attached.

These three steps are then repeated over and over until enough DNA has been copied. By making these copies, scientists are able to analyze DNA in detail, even if the amount of starting DNA is small. (Khan Academy has a great description of the PCR process here if you’re interested in more information.)
Reverse transcriptase PCR (RT-PCR) is a version of PCR that was developed for the measuring of RNA instead of DNA (2). RNA is basically a smaller (single stranded) version of DNA which is portable and used by the cell as a messenger of the DNA’s instructions. Using this method, we can determine the gene expression of a sample. Gene expression is information on the DNA as it is being made into a functional gene product, like a protein.
When you add in the ‘real-time’ part of rRT-PCR, this means that your results are essentially available in real time. This procedure allows you to see the copies generated during each cycle of the PCR process.
Testing COVID-19
You may remember that SARS-CoV-2 is an RNA virus. Therefore, it makes sense to test for its presence by looking for its viral RNA. This is why rRT-PCR is being utilized for patient testing. Scientists already know what the SARS-CoV-2 RNA looks like, so they are able to test for its presence.
However, testing in the US started off very slow and you may have seen there were a lack of tests available. This is because at the beginning of the outbreak in the US, the Centers for Disease Control (CDC) was the only laboratory with the primers and reaction mixtures to be able to run the test. Therefore, when a Point-of-Care facility took a sample from a patient, the sample had to be correctly stored (refrigerator or freezer), correctly shipped (on ice or dry ice), and then examined at the CDC. This greatly slowed down the process.

This slow down changed on February 4th, 2020, when the Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS) issued what is called an emergency use authorization (EUA) determination. An EUA determination is issued when, “there is a significant potential for a public health emergency that has a significant potential to affect national security or the health and security of United States citizens (3).” The EUA designation allows for the use of unapproved medical products or unapproved uses of approved medical products for treating of life-threatening diseases (4). Under this authorization, private labs were able to start making testing kits that did not need full testing to be approved by the FDA for use. The CDC also started distributing kits allowing for Point-of-Care facilities to start testing in house. A lab can then apply for an EUA to be able to distribute their test.
As of April 1, 2020, the FDA has issued EUA authorization to 25 tests for the diagnosis of COVID-19. (You can find this list here.)
Antibody Based Tests
But, I did mention that there were two types of tests being developed for COVID-19. The second test type, which has been discussed recently in the news, is an antibody based test. Unlike rRT-PCR which can take hours to run, antibody based tests can be done in as little as 15 minutes.
The Technology
In your body you have two ‘types’ of immune system responses. The first is called your innate immunity (5). Innate immunity refers to the mechanisms your body already possesses to fight off an infection such as: your skin as a physical barrier, tears to keep particles out of your eyes, and inflammation in response to a wound. The second, and what we are concerned with here, is called your acquired or adaptive immunity (6). Adaptive immunity refers to the mechanisms your body uses to ‘learn’ from experience.
A major way that your adaptive immune system works is through the production of antibodies. An antibody is a Y-shaped protein that gets produced by your immune system after it is exposed to a foreign object or “antigen.” This antigen is often a bacteria or virus that makes you sick. Once an antibody is produced, the body then can remember the foreign object if it comes into contact with it again. The second time around, because your body has learned, the body is able to kill the antigen quicker, often without you getting sick.
This production of antibodies is what people are referring to when they say someone is immune to a disease. The antibody protein works by being able to attach to the antigen when it comes into contact with it. They are very specific and will only bind and recognize the antigen for which it was made after.
Tests that utilize antibodies work through a technique similar to enzyme-linked immunosorbent assays (ELISAs). While there are different types of ELISAs, they generally work through the same principles (7, 8):
- An antibody is coated on a plate that is specific for the virus (or other antigen) you are looking for. This is called the capture antibody.
- Samples are added to the plate and, if the antigen is present, bind to the capture antibodies.
- A second antibody is added to the plate that has a tag attached to it. This tag allows us to view if there was antigen present, such as creating a color in the sample. This is called the detection antibody.
- An enzyme is then added to the plate which binds with the detection antibody.
- Finally, a solution is added that induces a color change when it is altered by the enzyme.
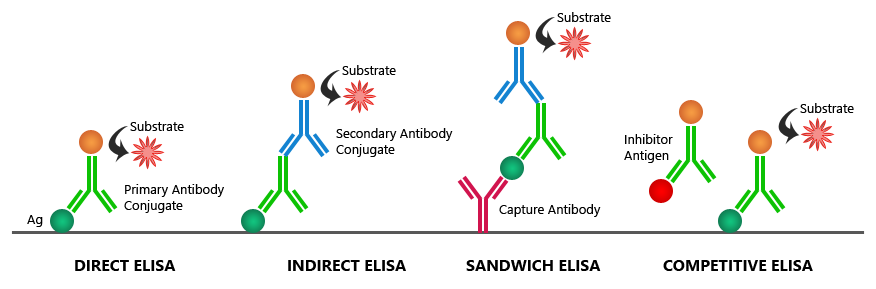
The resulting color change can tell us visually if the antigen is present or not in the sample.
In similar ways, we can detect if an antibody is present in a patients serum. Serum is the part of blood that remains after the blood cells are removed. These tests would have fragments of the virus where a person’s antibodies, if present, could bind to and be detected (9).
Testing COVID-19
In theory this idea seems like the perfect solution. Antibody testing would allow for in-home kits that would allow you to determine if you possessed antibodies against SARS-CoV-2. This would be ideal for patients that did not meet the criteria at Point-of-Care facilities to receive a test. It is important to keep in mind though, that unlike PCR strategies that tell you if a patient is currently infected, antibody tests can only tell you if a patient has antibodies, and not if they are infectious.
Additionally, production of specific antibodies are difficult as antibodies are not easily synthesized in a laboratory setting. Antibody production is often done through the use of laboratory or farm animals who get injections of the antigen (in this case SARS-CoV-2) and then produce antibodies that we can collect for use.
Another problem with antibody tests is sometimes they aren’t as specific. For example, if a patient had antibodies against another coronavirus, they could test positive in an antibody test for SARS-CoV-2. Finally, detection limits are also an issue with antibody tests. There needs to be enough of the antibody present in the blood that the test can recognize it. It takes time for the body to create antibodies after contact with an antigen which can add to the test’s inaccuracy.
Takeaways
- Currently, there is one approved test type on the market in the US. It utilizes something called real-time reverse transcriptase polymerase chain reaction (rRT-PCR) to make copies of viral RNA so that it can be detected.
- These tests were originally only being done at the Centers for Disease Control (CDC) headquarters which slowed down testing.
- The FDA issued an Emergency Use Authorization (EUA) declaration that allowed for the use of unapproved medical equipment and tests. This allowed private labs to create tests that could be done at Point-of-Care facilities utilizing this technology.
- New antibody based tests are being developed with methods similar to enzyme-linked immunosorbent assays (ELISAs) which could be done in as little as 15 minutes.
- Antibody tests can tell you if you have antibodies because you have previously encountered the virus. They cannot tell you if you are infectious.
- Antibody tests are also more difficult to create and are less accurate than rRT-PCR tests.
Overall, I hope this gave you some information on how tests for COVID-19 work and are being used. It is suspected as we have more tests available, we will see more infections. This includes those who has mild cases of COVID-19 without even knowing it.
As always, your best bet is to stay up to date on the science and stay home. We will all get through this.
Some Helpful Sources:
- COVID19 Global Spread, interactive maps – here
- CDC testing statistics by state – here
- CDC approved samples for PCR tests – here
- FDA approved EUAs – here
- Article on antibody test development – here




2 COMMENTS
Heide
5 years agoHi Allie (again)!
This one is great and is going to be assigned to my AP Bio students to read and respond to this week. You write beautifully – with clarity and accuracy. Did you ever imagine that you would be in a position where your old AP bio teacher would be including your work as assignments to current AP students? Very cool!
The Pretty PhD
5 years ago AUTHORThanks Heidi,
That’s such an honor! I’m glad my articles are useful. That’s what I’m aiming for! 🙂